How Do Motor Cortex Pathways Change in Aging?
Post by Rebecca Glisson
The takeaway
As we age, we sometimes lose our ability to move normally, which also significantly lowers our quality of life and capacity for independence. The motor cortex of the brain, which controls our movement, and the brain pathways descending from this area deteriorate in older adults, suggesting a focus on these areas could help us better treat neurodegenerative diseases.
What's the science?
Our movement is controlled by an area of the brain called the motor cortex, which can lose its function over time as we age. The motor cortex sends signals through two tracts, or pathways, of cells: the corticospinal tract (CST) and the corticostriatal tract (CStrT). This week in NeuroImage, Wen and colleagues investigated the changes in the CStrT that occur as people age, aiming better to understand the neural basis of movement-related neurodegenerative diseases.
How did they do it?
The authors wanted to study how aging affects several parts of the motor cortex and its pathways: its overall structure, how blood flows through this area, and the quality of the cells in these areas. To study these variables related to movement abilities, the authors used structural magnetic resonance imaging (MRI) to image and analyze the motor cortex in the brain. They also used diffusion MRI to analyze the pathways of the CST and CStrT. Linking this to movement, the authors measured participants’ motor function using endurance and locomotion walking tests and grip strength tests. The authors grouped participants into two age groups: the younger group, 36 to 65 years old, and the older group, 66 to 90 years old.
What did they find?
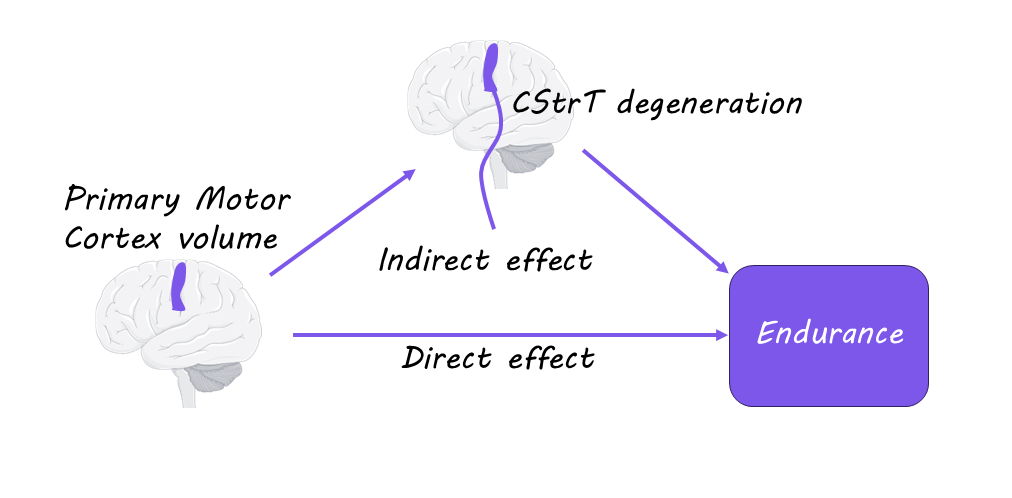
While younger participants had normal brain structure, the authors found that older participants had less volume in the motor cortex and less blood flow to this region. The older group of participants also had significantly lower movement abilities involving locomotion, endurance, and strength. This suggests that the loss of certain movement abilities as we age is due to degeneration of the motor cortex. The authors also found that the CST and CStrT pathways were of significantly lower quality in the older group of participants and that this deterioration mediated the relationship between motor cortex atrophy and decline in motor function. Therefore, the CST and CStrT pathways are particularly important to movement function and are affected in aging.
What's the impact?
This study is the first to show that the changes in the motor cortex and its related pathways in the brain due to aging are directly related to a loss of movement functioning in older adults. This highlights the need to focus on these areas for studying movement diseases related to aging. Studies like these can help us detect neurodegenerative disorders earlier and develop better and more effective treatments.